An autism gene regulates a female-specific phenomenon
While it is clear that Autism Spectrum Disorders (ASD) are complex genetic conditions, the biological mechanisms leading to ASD not clearly understood as yet. Recent studies have identified a gene called Chd8 (referring to the gene name) whose mutations lead to autism-like symptoms and enlarged brain size (hyperplasia).
CHD8 is a protein, namely a transcription factor (or TF), which means it works by regulating the production of other proteins through an RNA intermediate – a molecule containing the information encoded in the DNA itself. In order to produce a protein, a gene passes through an intermediate medium called RNA. Some genes do not produce a protein and are functional as RNA.
CHD8 and Xist in women
While the mechanisms leading to ASD are unknown, there is an interesting observation about the protein CHD8. Many genes (more than 2000) have been found to depend on CHD8 for their correct expression, although their dependence on CHD8 is not inversely proportional to the abundance of CHD8 protein. Researchers found that, by reducing CHD8 levels down to about 35% of the normal amount, the brain increased in size. Beyond this point, if the levels of CHD8 were reduced to about 15%, there would be a reversal of the expected brain size change, as if there were a compensating mechanism kicking in. That being said, other symptoms or phenotypes emerged with a more severe depletion of CHD8.
A nonlinear effect of CHD8 has also been detected in the CHD8 regulation of a gene called Xist. This gene produces an RNA but not a protein. This RNA is known to carry out a very important function in women, known as ‘X chromosome inactivation’ (XCI). To put it simply, whereas men have one X and one Y chromosome, women have two X chromosomes. Having protein products from both X chromosomes would result in an excessive amount of many proteins and would be severely damaging to the organism. For this reason, one of the chromosomes has to be inactivated early on in embryonic development and Xist is the gene responsible for this inactivation.
How does CDH8 control the production of other proteins?
While we don’t have a definitive answer as to why and how CDH8 controls the production of other proteins in a non-linear way, we do have some answers from the X inactivation field. In particular, the authors proposed a model called ‘competitive binding’. In this model, CHD8 is not the only transcription factor driving Xist production but there is another one regulating Xist production with greater potency, called YY1. What is proposed is that when CHD8 is nominal or mildly reduced, CHD8 can still bind the Xist promoter (a region that controls how much of a gene becomes an RNA, the intermediate medium between DNA and proteins) causing a corresponding reduction of its abundance. However, when the levels drop further, it can no longer hold on to the promoter and is displaced by a more efficient TF (such as YY1) or yet to be discovered transcription factors. This would lead to the observed effect due to the associated increase in transcription.
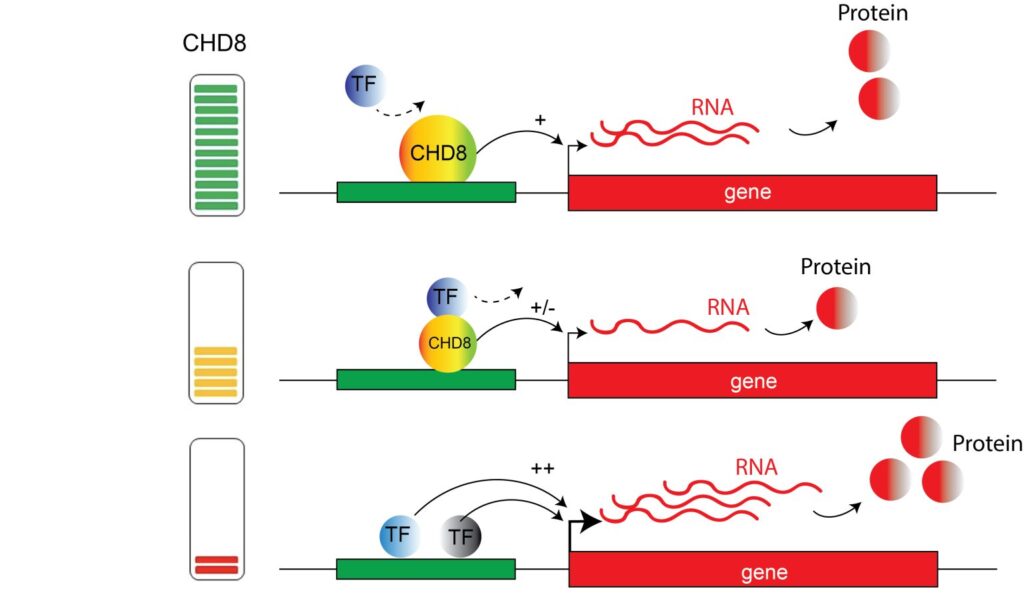
The model for this nonlinear effect of Chd8 on Xist is depicted in the main image of the article, shown above. As shown in the top panel, when CHD8 is at 100%, it contributes to the proper regulation of gene expression. The middle panel shows that when CHD8 level is slightly reduced, it can still contribute to the regulation of gene expression, but the genes that are regulated by it are less expressed, and less protein is made. In the bottom panel, when CHD8 is completely depleted, other transcription factors can access genomic regions that are normally regulated by CHD8, causing the non-correct expression of these genes (ie, more or less protein is formed). Even though this particular case cannot explain the nonlinear effects on brain size (Xist is not expressed in males and males are more susceptible to autism) it is may be applicable to other CHD8 controlled genes which are in turn responsible for the observed brain hyperplasia and Chd8 mediated gene dysregulation.
Andrea Cerase (andrea.cerase@unipi.it), Giuseppe Trigiante (g.trigiante@kingston.ac.uk)
Email:
Phone number:
Institute:
University of Pisa, Kingston University
Address line 1:
Unit of Cell and Developmental Biology
Your City:
Pisa
Your Country or State:
Italy
Your Postal / Zip Code:
56123
References:
Hurley S, et al, (2021) Distinct, dosage-sensitive requirements for the autism-associated factor CHD8 during cortical development. Mol Autism, 24;12(1):16. doi.org/10.1186/s13229-020-00409-3 PMID: 33627187; PMCID: PMC7905672.
Cerase, A, et al, (2021) Chd8 regulates X chromosome inactivation in mouse through fine-tuning control of Xist expression. Commun Biol 4, 485, doi.org/10.1038/s42003-021-01945-1
